Isidor Buchmann, CEO and Founder, Cadex Electronics, Inc.
The leading health indicator of a battery is capacity; a measurement that represents energy storage. A new battery delivers (should deliver) 100 percent of the rated capacity. Lead acid starts at about 85 percent and increases in capacity through use before the long and gradual decrease begins. Lithium-ion (Li-Ion) starts at peak capacity and begins its decline immediately, albeit very slowly.
To reduce stress, charge Li-Ion with a moderate two to three-hour charge rather than an ultra-fast charge of less than one hour. Prevent harsh and erratic dischargers. It is better not to drain a battery fully but charge it more often. As the author of www.BatteryUniversity.com, I am citing these recommendations as they are part of the most commonly asked questions of this educational website on batteries.
Manufacturers base device specifications on a new battery, but this is only a temporary state that does not represent a battery well from cradle to grave. Performance will decrease with use and time and the loss will only become noticeable once the shine of a new device has worn off and daily routines are taken for granted. An analogy is an aging man whose endurance begins to fade after the most productive years draw to an end. Figure 1 demonstrates such an aging process.
When I ask battery users, “At what capacity do you replace the battery?” most reply in confusion, “I beg your pardon?” Few folks are familiar with the term capacity as a measurement of energy storage and runtime, and even less when to retire a pack. Performance loss only becomes apparent when breakdowns occur and the battery becomes a nuisance.
Battery retirement depends on the application. Organizations using battery analyzers typically set the capacity threshold for replacement to 80 percent. There are applications where the battery can be kept longer and a toss arises between “what if” and economics. Some scanning devices in warehouses can go as low as 60 percent and still provide a full day’s work. A starter battery in a car still cranks well at 40 percent. Engine-starting only requires a short discharge that is replenished, but letting the capacity go much lower may get the driver stranded without warning (more on this later). No one gets hurt if a faded battery cuts a phone call off, but a failing medical device can put a patient at risk. Running out of power in an industrial application can also incur high logistic costs.

The best indicator when to retire a battery is checking the spare capacity after a full shift. Battery analyzers do this by applying a discharge before charge. A battery should have 10 to 20 percent spare at the end of a day to cover unknowns and emergencies. If the lowest performing battery in the fleet comes back with 30 percent, then the target capacity can safely be lowered from 80 percent to 70 percent. Figure 2 illustrates a battery analyzer that measures the spare as well as the full capacity of a battery.
Let’s take a drone specified to fly for 60 minutes on a good battery. Unknown to mission control, the capacity may have dropped to 75 percent, reducing the flying time to 45 minutes. This could crash the $50,000 vehicle while attempting to negotiate a second landing. With the reserve capacity marked on each pack using the analyzer’s print option, batteries delivering close to 100 percent can be assigned for long hauls while older packs may be sent for shorter errands. This allows the full use of each battery and establishes a sound retirement policy based on application and experience.
Many batteries and portable devices include a fuel gauge. While this indicates dispersing of energy from full to empty; capacity estimation is sketchy. After a full charge, state-of-charge (SoC) always shows 100 percent whether the battery is new or faded. This creates a false sense of security by assuming that a fully charged battery would deliver the anticipated runtime. Furthermore, runtime data gets inaccurate through use and time, and the battery needs calibration. The instruction manual of an Apple iPad says, “For proper reporting of SoC, be sure to go through at least one full charge/discharge cycle per month.” Engineers call this “digital memory.”
The industry has been aware of deficiencies and since the 1990s device manufacturers added “smarts” to batteries. The SMBus, a widely used smart system, measures voltage and current during charge and discharge. While this provides SoC, cycle count, error diagnostics and more, SMBus cannot estimate capacity with certainty. The digital battery, which SMBus manages, is exclusively based on peripheral readings. Checking the chemical battery with a full discharge would be best but this is time consuming. Rapid-testing is desirable but the chemical battery is a very complex.
In the absence of maintenance, some device manufacturers mandate to replace a battery on a date-stamp or cycle count. A pack may fail before the appointed time but most last far longer, prompting perfectly good batteries to be discarded prematurely. Dr. Imre Gyuk, manager of the Energy Storage Research Program at DOE, said that “every year roughly one million usable lithium-ion batteries are sent in for recycling with most having a capacity of up to 80 percent.” Lack of suitable battery diagnostics also affects heathcare. An FDA survey says that “up to 50 percent of service calls in hospitals surveyed relate to battery issues.” Healthcare professionals at the Association for the Advancement of Medical Instruments say that “battery management emerged as a top 10 medical device challenge.”
Rapid-Test Methods that No Longer Work
Knowing the health of a battery is important, but no practical method exists that can quantify all conditions in a short, comprehensive test. State-of-health (SoH) cannot be measured per se, only estimated to various degrees of accuracies based on available symptoms. A battery behaves like a living organism that is swayed by conditions such as SoC, charge and discharge events, rest periods and age. A battery with low SoC behaves similarly to a pack exhibiting capacity loss and these symptoms become a blur when estimating runtime. Rapid-test methods must isolate mood swings and only capture characteristics that relate to battery SoH.
The market offers a wide choice of battery testers and most provide correct predictions on a good or dead battery. So does the user. The challenge is evaluating usage and age-related symptoms before performance degradation become noticeable. Most testers provide voltage and internal resistance, also known as impedance, but capacity estimation is beyond reach with most. Some device manufacturers promote capacity readout nevertheless and this confuses the industry into believing that multifaceted results are attainable with basic methods.
Charge: 1,500 mA to 4.2 V, 25°C
Discharge: 1,500 to 2.75 V, 25°C
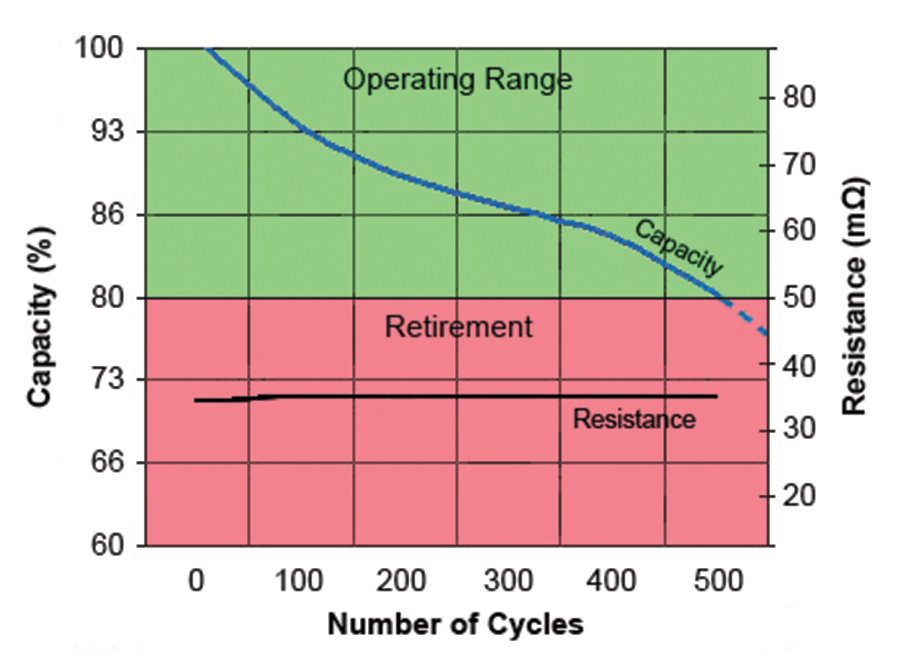
Early SoH testers linked capacity to internal resistance. While this one-size-fits-all approach worked reasonably well 10 years ago, modern lithium-ion and lead acid batteries have improved and retain low resistance. Figure 3 shows the relationship between capacity and internal resistance of modern Li-Ion as a function of cycle count. The chart shows a predictable capacity drops while the resistance stays flat throughout most of the service life. Lead acid has similar characteristics.
Credit for improved performance goes to advances in electrolytes and electrode materials, developments that reduce corrosion. But batteries still lose capacity and this is also apparent with modern cars where the starter battery has assumed added duties, such as start-stop. According to Allgemeiner Deutscher Automobil-Club, problems with starter battery have risen four times between 1996 and 2010. The report states that each third breakdown involves a discharged or defective battery; few packs reach the age of five years.
Discharging a fully charged battery would provide the most reliable capacity assessment but this is impractical for larger systems and requires removing the battery from service. There is also the stress factor. A lead acid permanently loses about two percent of its capacity with each full cycle; a starter battery fades as much as eight percent if fully discharged. Starter batteries should not be deep-cycled.
Most automotive battery testers measure cold cranking amp (CCA). CCA relates to internal resistance that tends to stay high while the capacity drops predictably with use and age. This gives the motorists a false sense of confidence by assuming that a good crank means a healthy battery. Cadex tested 175 aging starter batteries and found a correlation between CCA and capacity of only 0.55. A perfect match would be 1. Evaluating a starter battery on CCA readings is akin to tossing a coin.
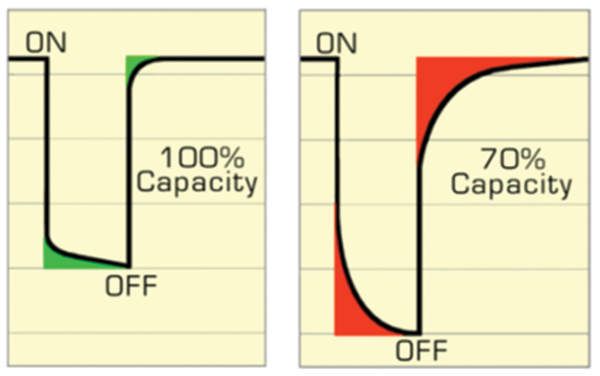
Battery rapid-testing is developing on two fronts and involves time domain with discharge pulses and frequency domain using electrochemical impedance spectroscopy (EIS). Cadex developed Electrochemical Dynamic Response (EDR) by observing voltage recovery on applied pulses. A fast recovery denotes good ion flow in Li-Ion; a sluggish reaction indicates capacity loss. An analogy is a dried-out felt pen that still writes but needs rest to replenish the ink. Some older batteries may also show large voltage deflection due to elevated internal resistance and EDR evaluates this condition as well. Figure 4 compares a good battery with quick recovery against a faded one with a sluggish response.
EDR is superior to regular pulse methods in that it can estimate SoH of Li-Ion, but the technology is ill-suited for larger batteries. EDR is also sensitive to the choice of electrolytes and cathode materials used. This can be solved by characterizing a battery, but the algorithm becomes battery specific. Research continues to find a more generic solution.
Technologies using frequency domain are almost always based on electrochemical impedance spectroscopy. The battery is scanned with a frequency spectrum from less than one hertz to several kilohertz. The high frequency reveals resistive qualities, also known as bird-eye view, but the battery’s treasures are hidden in the low frequencies.
Cadex has developed a multi-model electrochemical impedance spectroscopy, or Spectro for short. The battery is scanned to produce a Nyquist plot that characterizes the individual components of the Randles model. Randles represents a battery consisting of ohmic and reactive components. Electrochemical models, or matrices, are fitted to derive capacity and CCA values by executing some 40 million transactions. Matrices are lookup tables that bear resemblance with instruments reading letters, fingerprints and eye retinas.
The Spectro technology is embedded in the Spectro CA-12, a handheld battery tester that estimates the capacity and CCA in less than 30 seconds noninvasively. The battery must have a minimal charge of 60 percent. Best results are attained with a working battery taken from regular service. New batteries that lack formatting or have been in storage do not exhibit the same symptoms and the results can be less accurate than if taken form active batteries.
Another promising application for rapid-testing is integrating the health-check engine into a battery charger. Such a system will not only charge a battery but also assure a capacity of 80 to 100 percent when the ready light illuminates. A charger with the health-check feature provides quality control at no extra effort. It assists fleet managers in keeping only good batteries and showing the faded ones the exit door. Health-check will also enhance battery management systems (BMS) by adding capacity estimations. Most of today’s BMS only observe voltage, current and temperature.
Summary
Unlike a person who shows age marks with advancing years, batteries do not exhibit visible changes. They look similar when fully charged or empty, new or old, and in need of replacement. Batteries should receive the same treatment as a critical part on an aircraft, a medical device or an industrial machine where the replacement of a worn part falls under strict maintenance guidelines. Regulating authorities struggle to implement such procedures for batteries, but the lack of suitable test technology makes this almost impossible. This gives rise that bad batteries can hide comfortably among the good peers and enjoy immunity. Batteries escape the scrutiny of inspection and are labeled “uncontrollable.”
Battery analyzers are effective in maintaining smaller packs but battery users want rapid-test methods. Advancements are being made and scientists put great faith in EIS. In fact, Cadex’s own CA-12 battery tester is the first commercial application based on the EIS technology. While it has been around for many years, equipment size, high cost, long test times and the need for specialists to decipher the data made it prohibitive for the service end. Modern micro-electronics and advanced algorithm has moved the technology out of research laboratory into the hands of the ordinary service technicians.
EIS has the potential of delivering more, such as measuring SoC by impedance while the battery is being charged or is under a load, as well as quality control in battery manufacturing. Although programmers of apps are able to create stunning and believable readouts of SoC, and sometimes even capacity, the truth lies in the chemical battery. Peripheral readings of the chemical battery are only a faint mirror image of what is hidden in the guts; it’s the guts that must be conquered and not the superficial.
Isidor Buchmann is the founder and CEO of Cadex Electronics Inc. For three decades, Isidor has studied the behavior of rechargeable batteries in practical, everyday applications, has written award-winning articles including “Batteries in a Portable World”.
For more information, please visit www.cadex.com.







